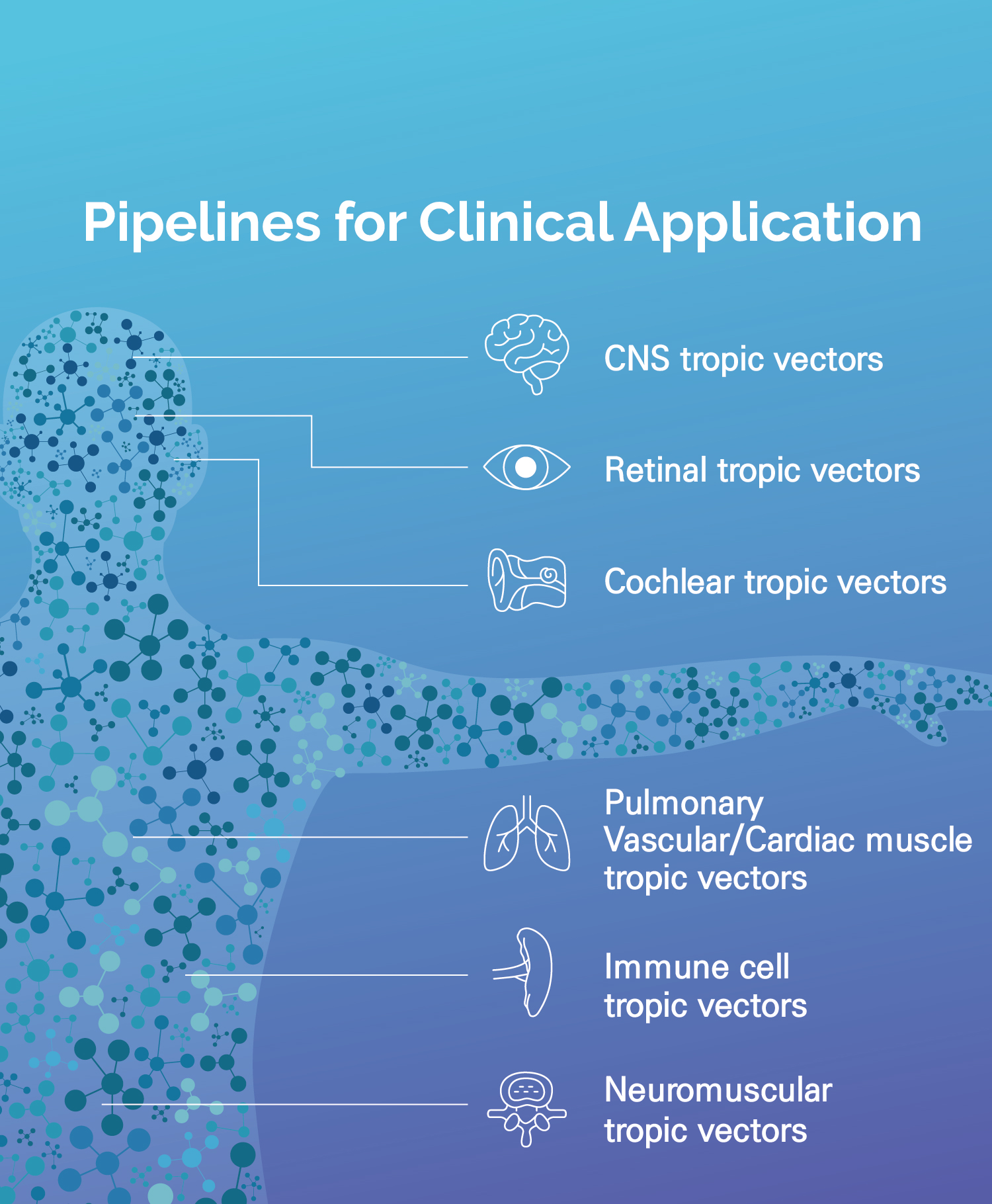
Featured Pipelines
GGT-100
An AAV vector (AAVp2CV) with evolutionary specificity for vascular smooth muscle cells and enhanced diffusion throughout the pulmonary system. It is being developed as a potential disease-modifying gene therapy for pulmonary arterial hypertension, a fatal disease with limited effective treatment options.
GGT-300
An AAV vector with evolutionary targeting specificity for astrocytes in the central nervous system, presenting next-generation gene therapy strategies applicable to a broad range of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.